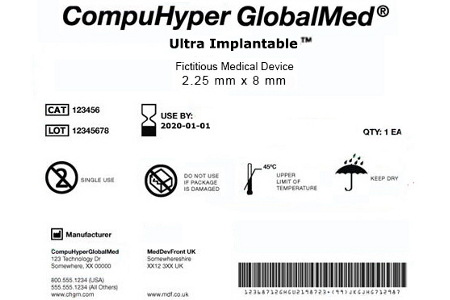
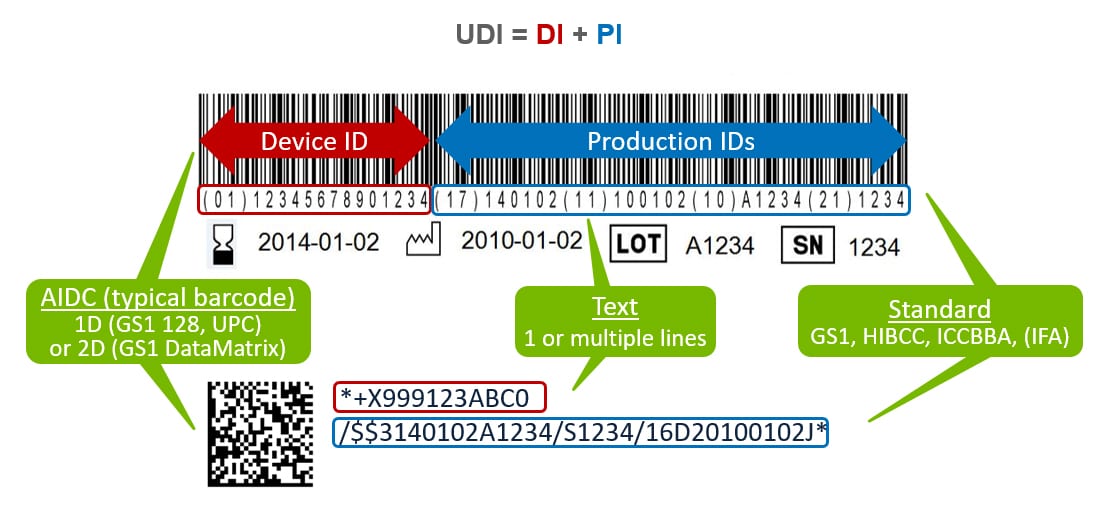
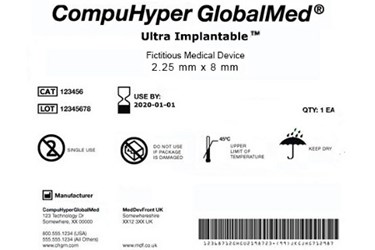
What do you need to know to comply with Phase 3 of FDA's Unique Device Identification System? - MedCity News

تويتر \ Topflight Corp. على تويتر: "Looking for UDI labels? Topflight provides top companies with UDI and prime label, as well as barcode and shipping labels. Talk with our team: https://t.co/1Yq11fzSC4 https://t.co/17gCL3Del1"

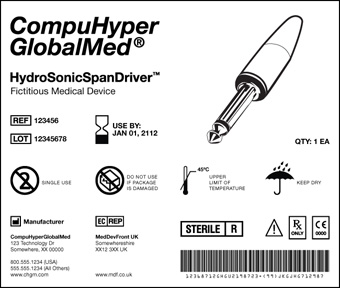




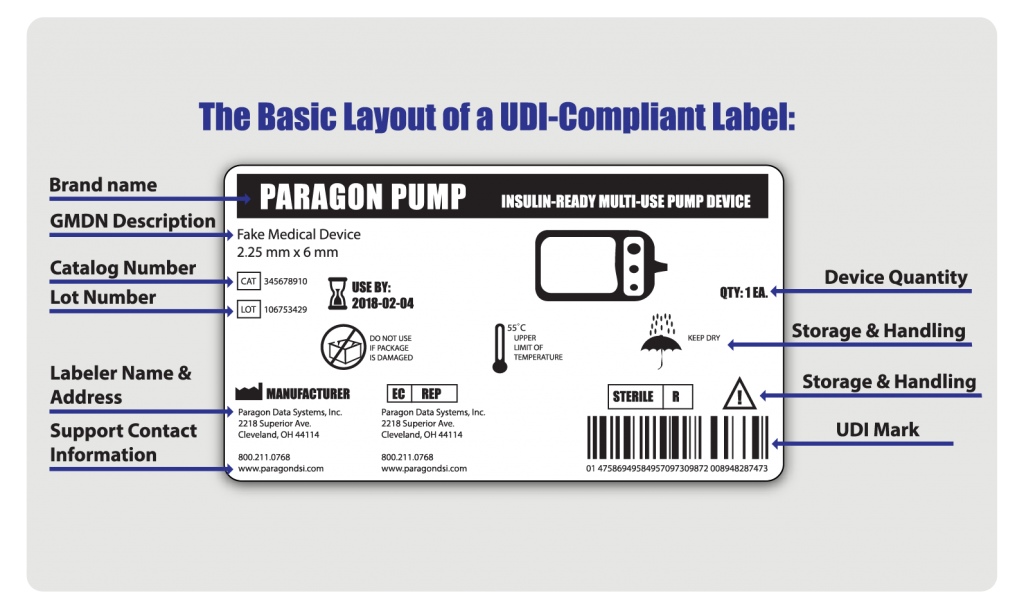


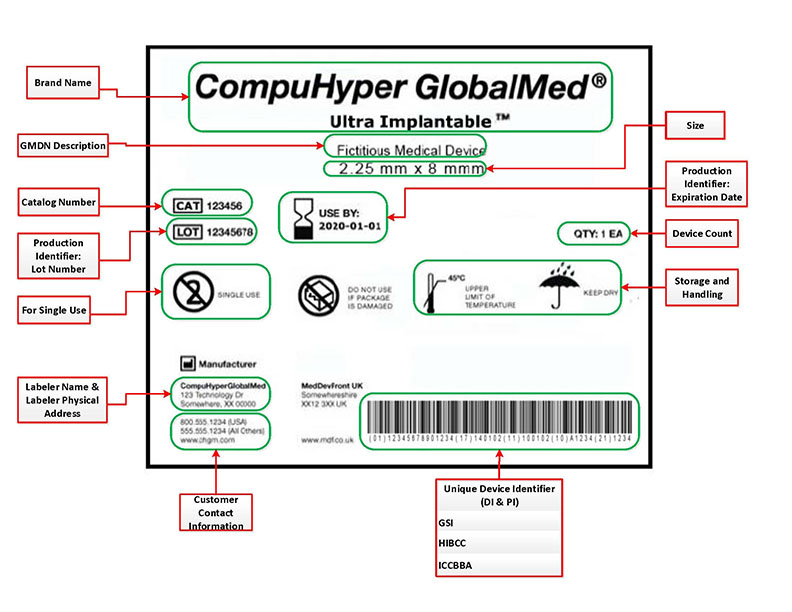
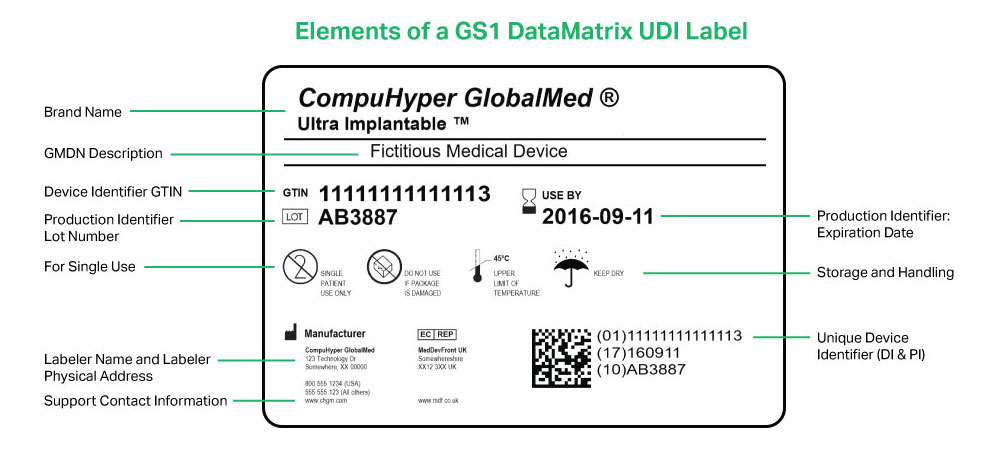
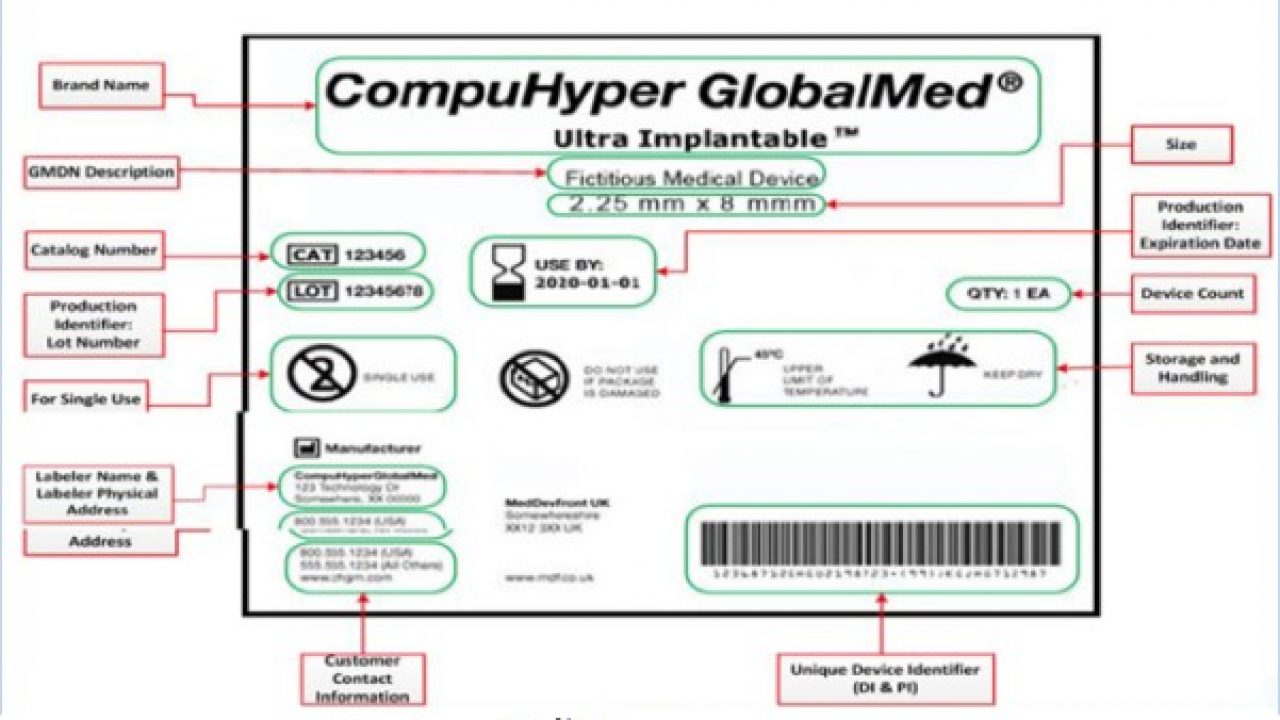
.png.aspx)